Compliance Infographics
While there is always some risk in working with select agents and toxins, our goal is to get as close to zero risk as possible. Unfortunately, incidents do occur, and sometimes serious deficiencies in biosafety or security measures are identified at the laboratories that work with these materials.
The good news is that the vast majority of laboratories are doing well in following the select agent regulations. However, when issues do arise, systems are in place to deal with those situations. Importantly, FSAP can take quick action in order to protect public, animal, and plant health, using a number of available options to address any potential risks and bring the entity back into compliance with the regulations.
The Federal Select Agent Program has developed a series of infographics in order to describe these processes in more detail.
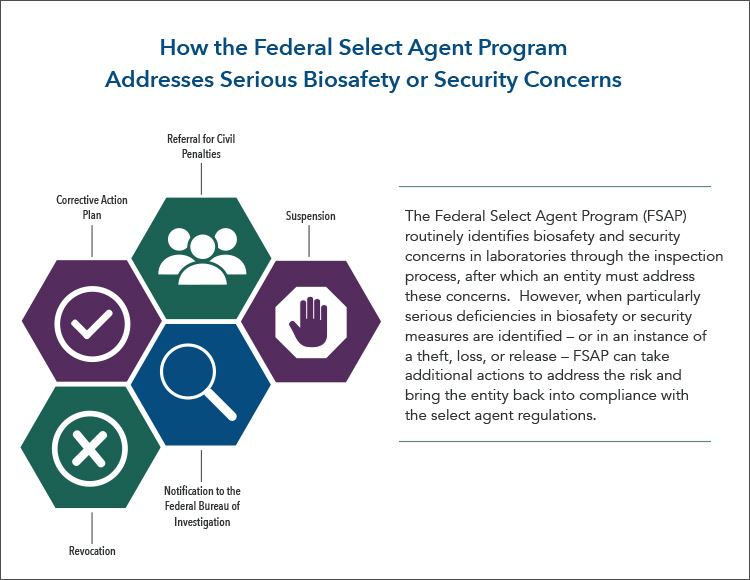
How the Federal Select Agent Program Addresses Serious Biosafety or Security Concerns
The Federal Select Agent Program (FSAP) routinely identifies biosafety and security concerns in laboratories through the inspection process, after which an entity must address these concerns. However, when particularly serious deficiencies in biosafety or security measures are identified – or in an instance of a theft, loss, or release – FSAP can take additional actions to address the risk and bring the entity back into compliance with the select agent regulations.
- Corrective Action Plan
- Referral for Civil Penalties
- Notification to the Federal Bureau of Investigation
- Suspension
- Revocation
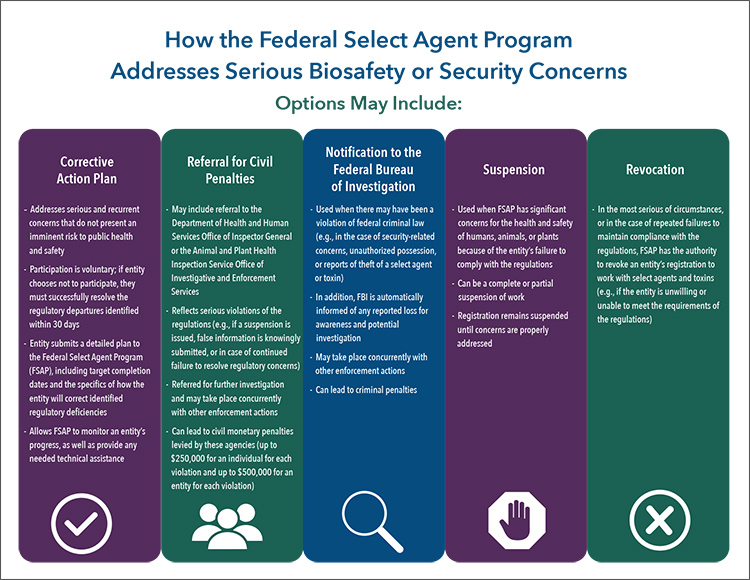
How the Federal Select Agent Program Addresses Serious Biosafety or Security Concerns
Options May Include:
Corrective Action Plan
- Addresses serious and recurrent concerns that do not present an imminent risk to public health and safety
- Participation is voluntary; if entity chooses not to participate, they must successfully resolve the regulatory departures identified within 30 days
- Entity submits a detailed plan to the Federal Select Agent Program (FSAP), including target completion dates and the specifics of how the entity will correct identified regulatory deficiencies
- Allows FSAP to monitor an entity’s progress, as well as provide any needed technical assistance
Referral for Civil Penalties
- May include referral to the Department of Health and Human Services Office of Inspector General or the Animal and Plant Health Inspection Service Office of Investigative and Enforcement Services
- Reflects serious violations of the regulations (e.g., if a suspension is issued, false information is knowingly submitted, or in case of continued failure to resolve regulatory concerns)
- Referred for further investigation and may take place concurrently with other enforcement actions
- Can lead to civil monetary penalties levied by these agencies (up to $250,000 for an individual for each violation and up to $500,000 for an entity for each violation)
Notification to the Federal Bureau of Investigation
- Used when there may have been a violation of federal criminal law (e.g., in the case of security-related concerns, unauthorized possession, or reports of theft of a select agent or toxin)
- In addition, FBI is automatically informed of any reported loss for awareness and potential investigation
- May take place concurrently with other enforcement actions
- Can lead to criminal penalties
Suspension
- Used when FSAP has significant concerns for the health and safety of humans, animals, or plants because of the entity’s failure to comply with the regulations
- Can be a complete or partial suspension of work
- Registration remains suspended until concerns are properly addressed
Revocation
- In the most serious of circumstances, or in the case of repeated failures to maintain compliance with the regulations, FSAP has the authority to revoke an entity’s registration to work with select agents and toxins (e.g., if the entity is unwilling or unable to meet the requirements of the regulations)
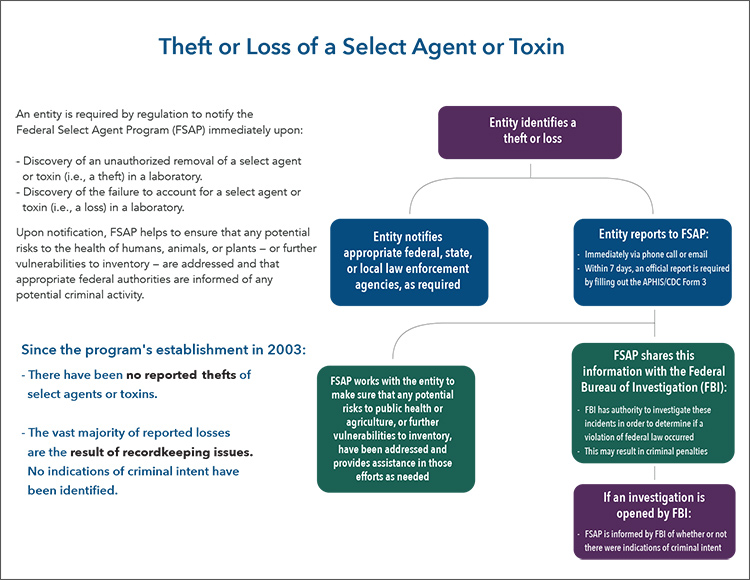
Theft or Loss of a Select Agent or Toxin
An entity is required by regulation to notify the Federal Select Agent Program (FSAP) immediately upon:
- Discovery of an unauthorized removal of a select agent or toxin (i.e., a theft) in a laboratory.
- Discovery of the failure to account for a select agent or toxin (i.e., a loss) in a laboratory.
Upon notification, FSAP helps to ensure that any potential risks to the health of humans, animals, or plants — or further vulnerabilities to inventory — are addressed and that appropriate federal authorities are informed of any potential criminal activity.
Since the program’s establishment in 2003:
- There have been no reported thefts of select agents or toxins.
- The vast majority of reported losses are the result of recordkeeping issues. No indications of criminal intent have been identified.
If an entity identifies a theft or loss:
- Entity notifies appropriate federal, state, or local law enforcement agencies, as required
- Entity reports to FSAP:
- Immediately via phone call or email
- Within 7 days, an official report is required by filling out the APHIS/CDC Form 3
- FSAP works with the entity to make sure that any potential risks to public health or agriculture, or further vulnerabilities to inventory, have been addressed and provides assistance in those efforts as needed
- FSAP shares this information with the Federal Bureau of Investigation (FBI):
- FBI has authority to investigate these incidents in order to determine if a violation of federal law occurred
- This may result in criminal penalties
- If an investigation is opened by FBI, FSAP is informed by FBI of whether or not there were indications of criminal intent.

Release of a Select Agent or Toxin
An entity is required by regulation to notify the Federal Select Agent Program (FSAP) immediately upon the discovery of the release of a select agent or toxin in a laboratory. Both entities registered with the program, as well as unregistered entities (such as clinical or diagnostic laboratories that may identify a select agent or toxin from a specimen for testing purposes), must immediately report this information.
While there is always some risk in working with these agents, our goal is to get as close to zero risk as possible. Unfortunately, sometimes incidents do occur, and this process helps to manage those situations.
What is a release?
- Occupational exposure (e.g., needle sticks, animal bites or scratches, failure of personal protective equipment)
- Release outside of the primary barriers of the biocontainment area (e.g., spill outside of a biologic safety cabinet or the laboratory itself)
What are the potential consequences?
-
- A release could result in potential exposure to and/or infection with a serious disease, either among individuals working in the laboratories or those in the surrounding communities. A release could also result in the infection of animals or plants or contaminate the environment.
- If an incident occurs and particularly serious deficiencies are identified, FSAP can take action as needed to address the risk and bring the entity back into compliance with the select agent regulations, including: suspending or revoking FSAP registration, or notification to federal enforcement agencies for consideration of civil or criminal penalties.
The majority of reported releases are potential occupational health exposures but do not result in illness.

Release of a Select Agent or Toxin: A Shared Responsibility
Who is Involved in a Response?
Entity Working with Select Agents or Toxins
- Laboratory experiences the release and must immediately report it to FSAP
- Critical that the entity immediately assesses the scope of the situation and the health of potentially exposed individuals, and ensures that the select agent or toxin is contained
- All registered entities are required to have an incident response plan as a condition of program registration
- This should include an assessment of risk and detail how the entity will handle those situations
- It should be specific to the entity and the agents they are working with, up-to-date, effective, and ready to implement when needed
State/Local Public Health or Agriculture Officials
- Responsible for human and agriculture health and safety in their jurisdiction, and have the authority and responsibility to assist the entity
- Can play an active role in assessing the situation and responding to the event
- Will typically engage when the situation goes beyond the ability of the entity to manage
- May request assistance from other federal agencies
Federal Select Agent Program (FSAP)
- Receives report of a release from entity
- Works with the entity to get a complete picture of the incident — primary focus is on making sure the threat to public health or agriculture is contained, any exposed individuals have been properly evaluated, the select agent or toxin is secure, and helping the entity to assess why the failure occurred so that it does not happen again
- Can facilitate assistance between the affected entity and appropriate agencies
- May conduct an on-site visit to confirm corrective actions have been taken and/or provide technical expertise to the entity
Other Federal Agencies
- May get involved in the case of a significant threat to public health or agriculture, or when specific assistance is requested
- Assistance is typically coordinated through the state’s emergency management process
- May include federal agencies such as the Environmental Protection Agency (EPA), Federal Emergency Management Agency (FEMA), or Federal Bureau of Investigation (FBI)

About the Federal Select Agent Program
Mandated by Congress, the Federal Select Agent Program (FSAP) regulates the possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public, animal or plant health, or to animal or plant products.
The program is managed jointly by the Centers for Disease Control and Prevention (part of the U.S. Department of Health and Human Services) and the Animal and Plant Health Inspection Service (part of the U.S. Department of Agriculture).
For more information, visit https://selectagents.gov/.
Key functions include:
- Develop regulations
- Implement regulations
- Enforce regulations